This page was generated from `/home/lectures/exp3/source/notebooks/L15/Dispersion and Absorption.ipynb`_.
Dispersion and Absorption¶
We would like to have a closer look at what happens with our atom in the electric field of a light wave. First of all, our toy atom is very small (around 0.1 nm) which is much smaller than the wavelength of ligth (500 nm). Thus the electric field appears to homogeneous over the cross section of the atom and seems to change over time with
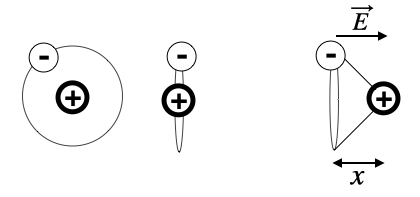
Fig.: Polarization of an electric cloud of an atom.
Lets assume the electric field being aligned with the x-direction, then we can write down the equation of motion for the displacement of the two charges in the atom
which is the equation of motion for a damped driven harmonic oscillator. Using our knowledge from the mechanical oscillator and the drivig electric field, we can use a solution
and insert that into the equation of motion to get the amplitude
This time dependent displacement creates a dipole moment
from which we may infer the electronic suceptibility
with
The susceptibility is therefore a complex number, which may write as
For the refractive index we can therefore write
where the minus sign between the real and imaginary part is a definition and sometimes handled differently. For the refractive index we may therefore write
The refractive index therefore has a real and imaginary part, which we can derive from the above formuala. We would like to a have a look at the consequences of those two coefficients
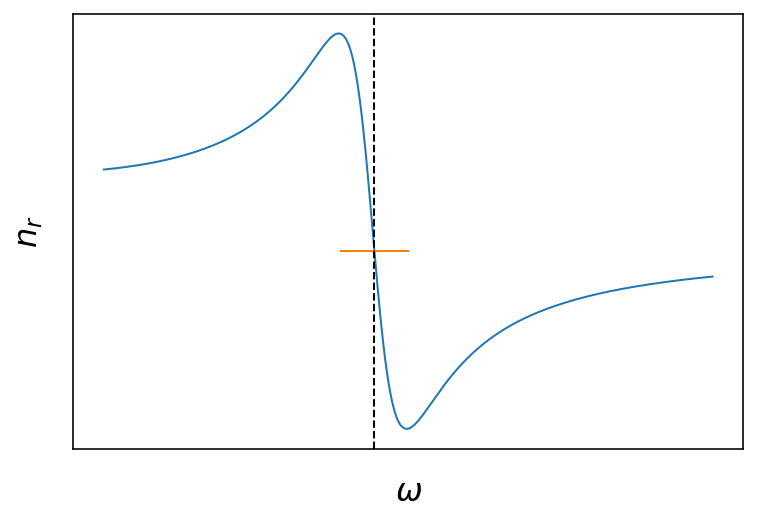
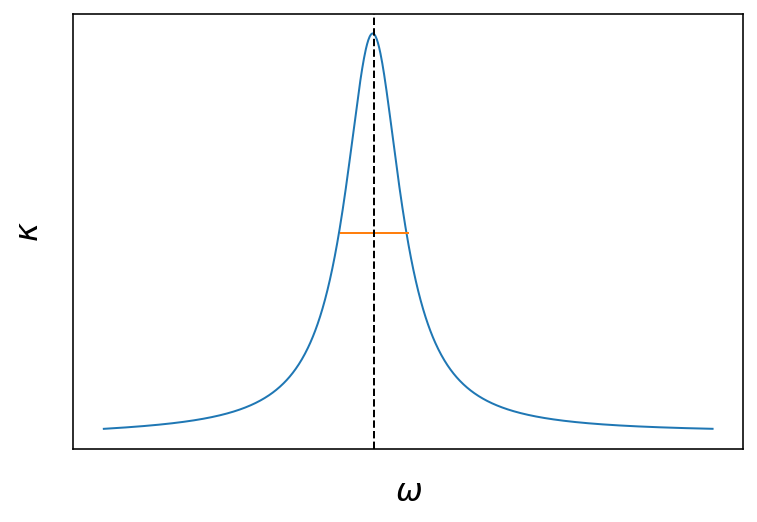
Fig.: Real and imaginary part of the refractive index as a function of the frequency. The vertical dashed line is located at
The two plots above show the frequency dependence of the real and imaginary part of the refractive index. The freuency dependence arises from the coupling to the resonance of the damped harmonic oscillator of our crude atomic model . Around the resonance, the are strong changes to be expected for the real part and the imaginary part. The real part has a dispersive shape being larger than 1 at lower frequencies and smaller than one (!) above the resonance. The imaginary part has the shape of a
Lorentzian curve around the resonance frequency, where the full width at half maximum (FWHM) of the curve us given by the damping coefficient
Absorption¶
We will first analyze the effect of
Inserting the complex refractive index yields
The imaginary part is creating a new real factor, which is inducating an electric field amplitude, that decays with the distance.
If we calculate the intensity we find
where
is called Lambert-Beers law.
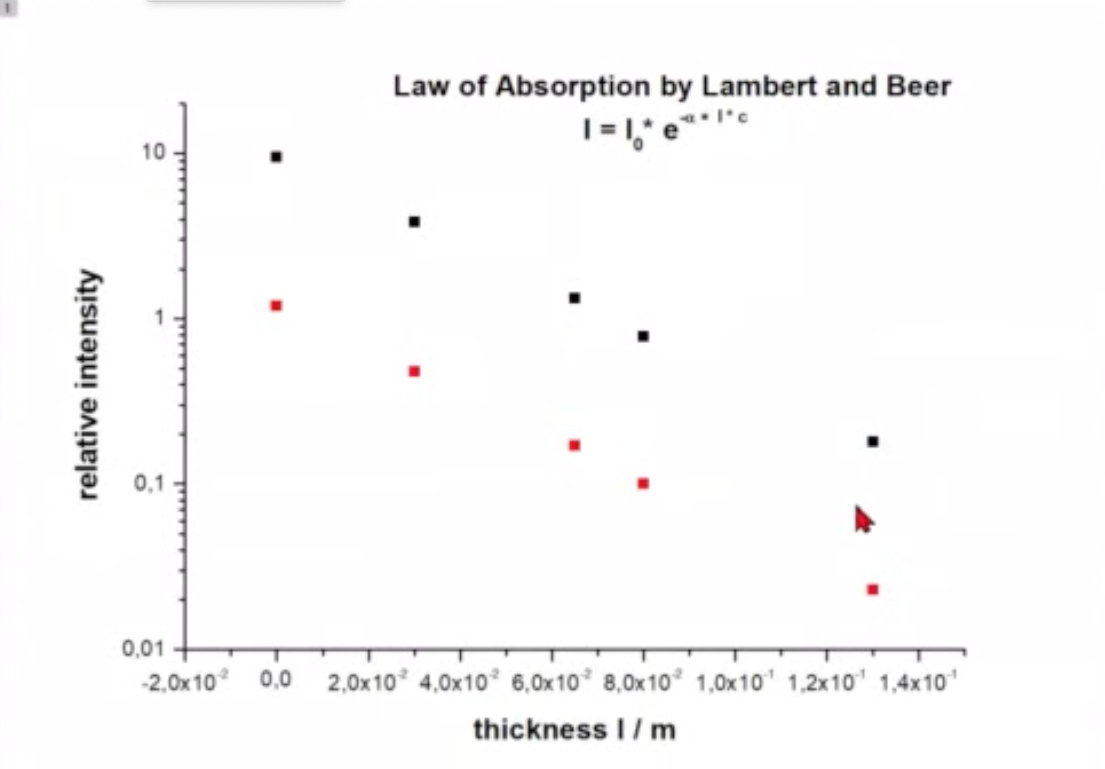
Fig.: .
As the coefficient
Dispersion¶
Besides the frequency dependence of the imaginary part of the refractive index, the real part has also a very pronanounced feature. This has consequences for the propagation speed of the wavefronts (phase velocity) or the speed of whole groups of waves, which is described by the group velocity
We know already the definition of the phase velocity
The group velocity is given by
The latter can be related to the refractive index
With the help of
The derivative of the refractive index by the wavenumber can be obtained when looking at the total differebtial
which gives
The inverse of this expression can now be inserted in the group velocity and finally yields
Based on this equation, we may characterize the dispersion, i.e. the variation of the refractive index with the frequency. If the coefficient
is positive, we call this normal dispersion. This is what we observed for the refractive index when discussing the prism or the rainbow. The refractive index is larger for shorter wavelength then.
In the case when
we call this anomalous dispersion. In both cases we see the the change of the refractive index of materials with the frequency is related to the polarizability of the material and the specific resonances. Yetm our model has been very crude.
What we may also find is a situation when the group velocity is larger than the speed of light. For this to occur we need to have
which is true for our susceptibility model at
This might seem to contradict our assumption of having nothing which can move faster than the speed of light. However, this assumption is only valid for the transport of energy, matter or information, not for the group velocity.
The transport of energy given by
is always smaller or equal to the speed of light.
