This page was generated from `/home/lectures/exp3/source/notebooks/L22_AMA/Particle_character_of_light.ipynb`_.
The Particle Character of Light¶
The photoelectric effect¶
In 1888 Wilhelm Hallwachs published an experiment with charged foils connected to a metal plate which was irradiated with ultraviolet light (we did this experiment in the last lecture before Christmas). If the foils and the plate are negatively charged and electrically isolated against the surrounding, the charge do decrease upon irradiation with ultraviolet light. In constrast, if the system is positively charged, the charge does not decrease. Hallwachs concluded that the light is responsible for negative charges leaving the metal plate.
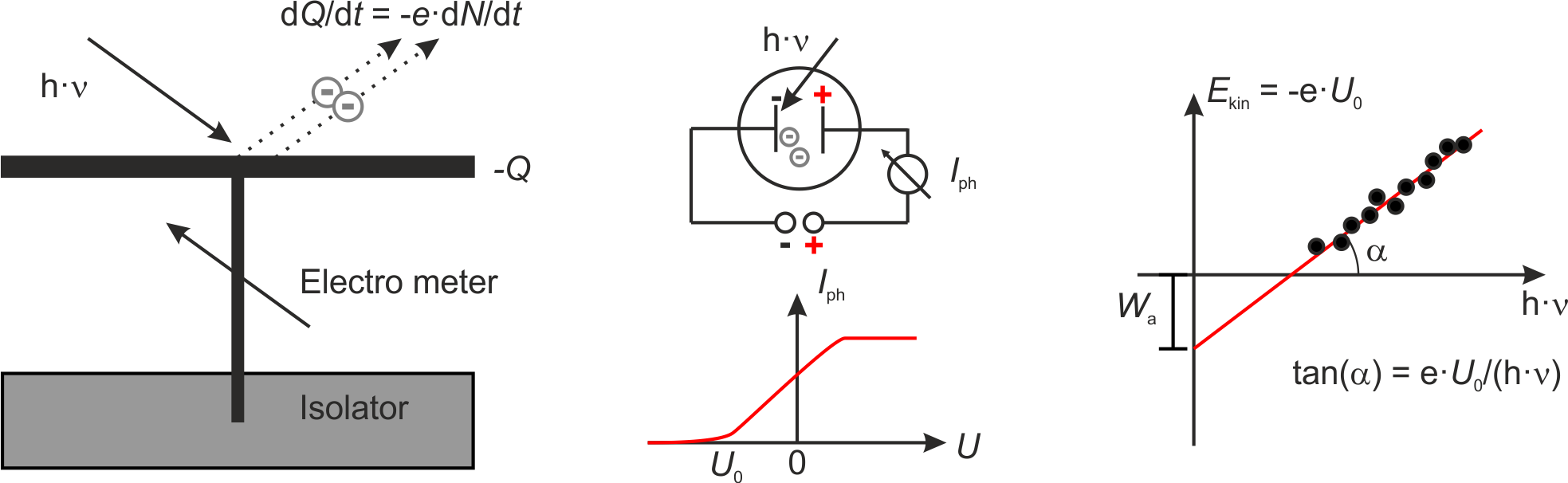
Fig.: (left) Scheme of the apparatus used by Hallwachs. (center) Scheme of the apparatus used by Lennard and the corresponding photocurrent :math:`I_{mathrm{ph}}`. (right) From the onset voltage :math:`U_0` one can calculate the work function :math:`W_{mathrm{a}}` as intercept and Planck’s constant :math:`h` as part of the slope.
Later in 1902 Lennard measured the photocurrent between two plates in vacuum. The current set in already at a negative voltage
The electrons must bear a minimum energy in order to overcome the oppositely directed electric field,
The kinetic energy
The number of photoelectrons is proportional to the light’s intensity.
There is no delay between light irradiation and electron emission.
In 1905 Einstein successfully explained these findings on the basis of the quantum model of light. In accord to this model every absorbed photon transfers its energy
with
Since one can determine the maximum kinetric energy
one is able to determine the work function on teh basis of the intercept of the
If we assume (against one’s better knowledge) a fully wave-like behavior of light, then light with a radiation power of
within the time interval
Let us consider a zinc plat with a work function of
reaching the plate. For a penetration depth of
electron, whereas each electron acquires on average a power of
Thus, it will take a time of
One example for an experiment proving Einstein’s explanation of the photoeffect was provided by von Joffé und Dobronrawov in 1925. They used small, charged bismuth beads holded within a Millikan capacitor and irradiated those beads with low-dose X-rays. Every change of the overall charge of the beads interfere the equilibrium in the capacitor, and can be observed by means of a change of the bead position. Using a radiation power of
The Compton effect¶
Another example for demonstrating the corpuscle (particle-like) character of light is the Compton effect. If an arbitrary material is irradiated with X-rays of wavelength
This phenomenon can be explained by means of the photon model and inelastic impact. Every photon bears an energy of
energy and momentum are conserved. The law of conservation of energy then reads as
with
and
with
to the photon, we can formulate teh law of momentum conservation as follow,
with
If we isolate the square of the momentum of the electron, we obtain an equation depending of teh squared difference between the wavevectors of the incident and scattered photon. Calculating this difference leads to a scalar product between these two vectors and necessitates the introduction of the angle between the propagation direction of the incident and scattered photon. We denote thsi angle as
From the law of energy conservation we get
which we can compare with the law of momentum conservation and get
Now making use of
with
The Compton wavelength is a constant and represents the change of the wavelength
represents the ratio between the energy of the incident photon and the energy of the electron at rest. Thus, if we know the mass of the electron, we can detremine
Properties of photons¶
During the 18th century there was a dispute about the nature of light. Newton proposed a particle-like character on the basis of the straight propagation and the law of refraction. In contrast, Huygens proposed a wave-like character of light on the basis of interference and diffraction. This interpretation seemed to be proven when Heinrich Hertz discovered electromagnetic waves and light was interpreted as a special spectral region being as well governed by Maxwell’s equations. As we have seen on the basis of the experiemnts, light –and more general electromagnetic waves– might show corpuscular characteristics as well. That is why we have to introduce a particle-like description in additon to the allready known wave-like description.
Energy and momentum¶
Every electromagnetic field consists of quanta of energy
We can further comprehend the flux
with
As demonstrated on the basis of the Comptopn effect, every photon bears a momentum
Then, the relation between the energy density
Angular momentum¶
Concerning the angular momentum of photons, if a free atom absorbs a photon, the angular momentum of the atom is altered by
Linearly polarized light can be comprehended as superposition of
Mass and gravitational force¶
From theory of relativity we know that the mass is actually affected through the traveling speed of the reference systems, namely
Furthermore, it is evedent that only particles with a mass at rest of
in accordance with energy and momentum considerations stated above. If we now ascribe a mass
and let this photon travel within a gravitational field, then the photon has to perform work in order to change its position from
For reasons of conservation of energy, the energy of the photon
and
Even though a photon does not have any mass at rest, a photon experiences a red shift (longer wavelength, smaller frequency, smaller energy), if it rises within a gravitational field. The energy decrease of the photon is equal to an increase in potential energy of
