This page was generated from `/home/lectures/exp3/source/notebooks/L28_AMA/L28_spherical_potential.ipynb`_.
A particle in a spherically symmetric potential¶
The sperically symmetric Schrödinger eqution¶
Now we would like to discuss how stationary states of a matter wave look like in a potential that exhibits sperical symmetry (
with the Laplace operator
The solution of the Schrödinger equation in the case of spherically symmetric potentials is easier, if we express this equation in sperical coordinates,
The Laplace operator in sperical coordinates then reads as
and we can reform the Schrödinger equation resulting in
In order to solve the time-indepedent Schrödinger equation we make use of separation of variables as we did before and use the ansatz
Using this ansatz in the last form of the Schrödinger equation results in
which we multiply by
The very last equation can be re-written in the form
which allows us to compared both sides. The left hand side of the last equation depends solely on
Solving the solution function
So far we have used the ansatz of separation of variables and reshaped the Schrödinger equation leading to the situation that both sides of the same equation depend on different variables. Because this equation is supposed to be valid for every values of
which has the solution
Since
Thus,
Moreover, we can normalize the solution in accord to
with the consequence for the normalization constant
Now we can state the normalized function
In addition, these functions are normalized, namely
Solving the solution function
In order to solve the solution for
and deivide it through
Similar to the case of the function
In the case
The solution of this differential equation we set in form of a power series
Moreover, in order to obtain finite values for the series especially in the case of
Since the series has to be finite, we set the
The solutions of the Legendre differential equation are the Legendre polynomials (similar to the Hermite differential equation and Hermite polynomials),
Since the propability density
In the case
Since
follows immediately. The constant prefactor in
The product functions
are called sperical harmonics. Since both factors are allready normalized it immediately follows for the product
The square of the product function
The normalized spherical harmonics up to
0 |
0 |
|
1 |
||
1 |
0 |
|
2 |
||
2 |
||
2 |
0 |
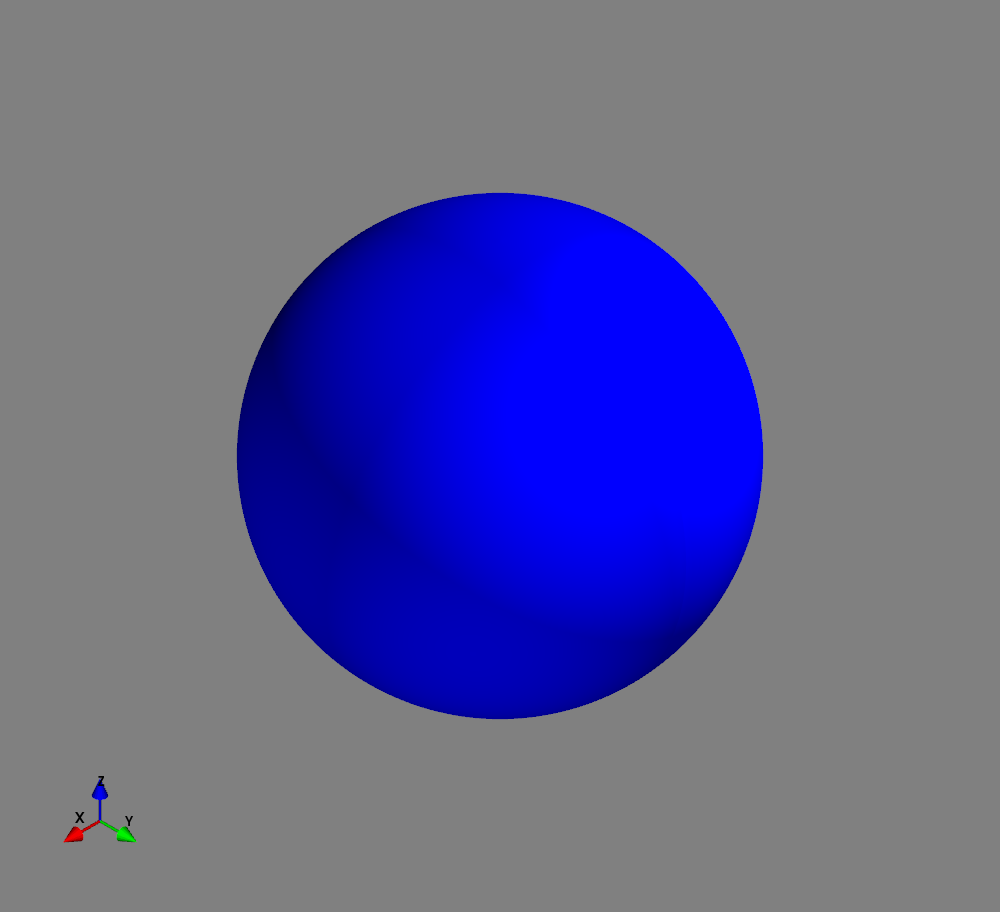
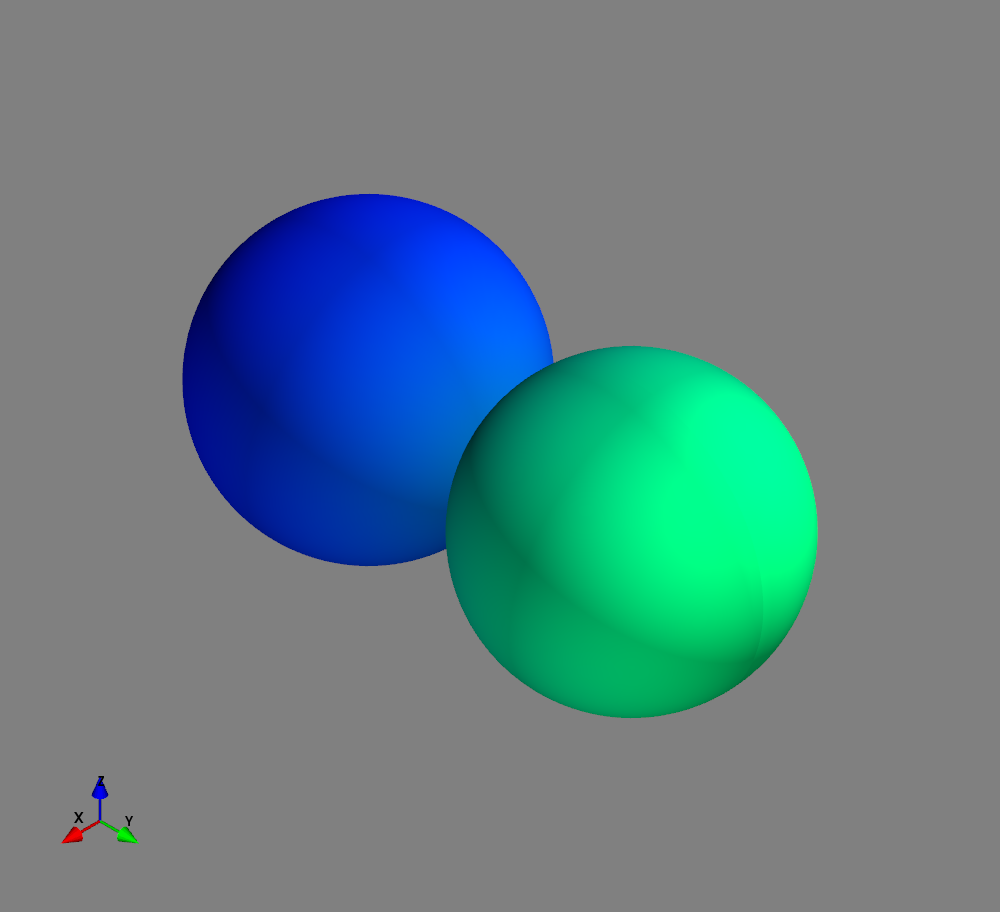
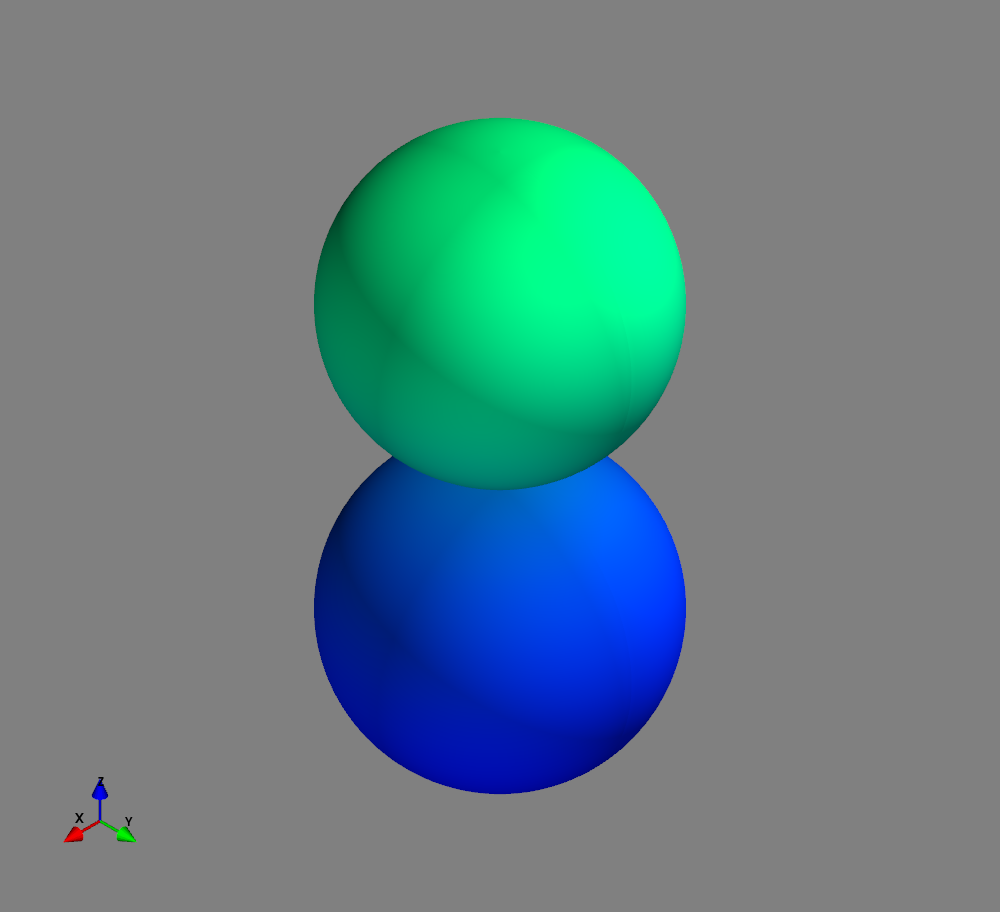
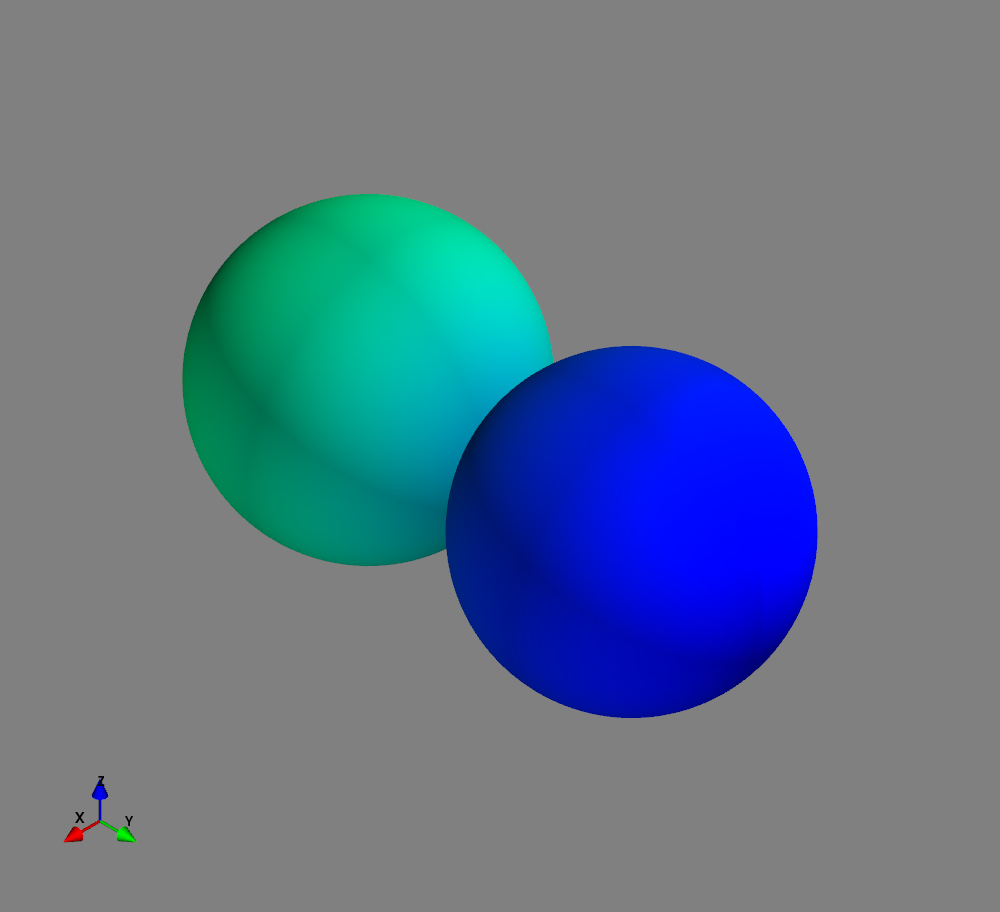
Fig.: Spherical harmonics :math:`Y_l^m left(vartheta, varphiright)` from left to the right: :math:`Y_{0}^{0}`, :math:`Y_{1}^{-1}`, :math:`Y_{1}^{0}`, and :math:`Y_{1}^{1}`. Note the swaped colors of the dumbbells, if changing :math:`m = -1 rightarrow +1`.
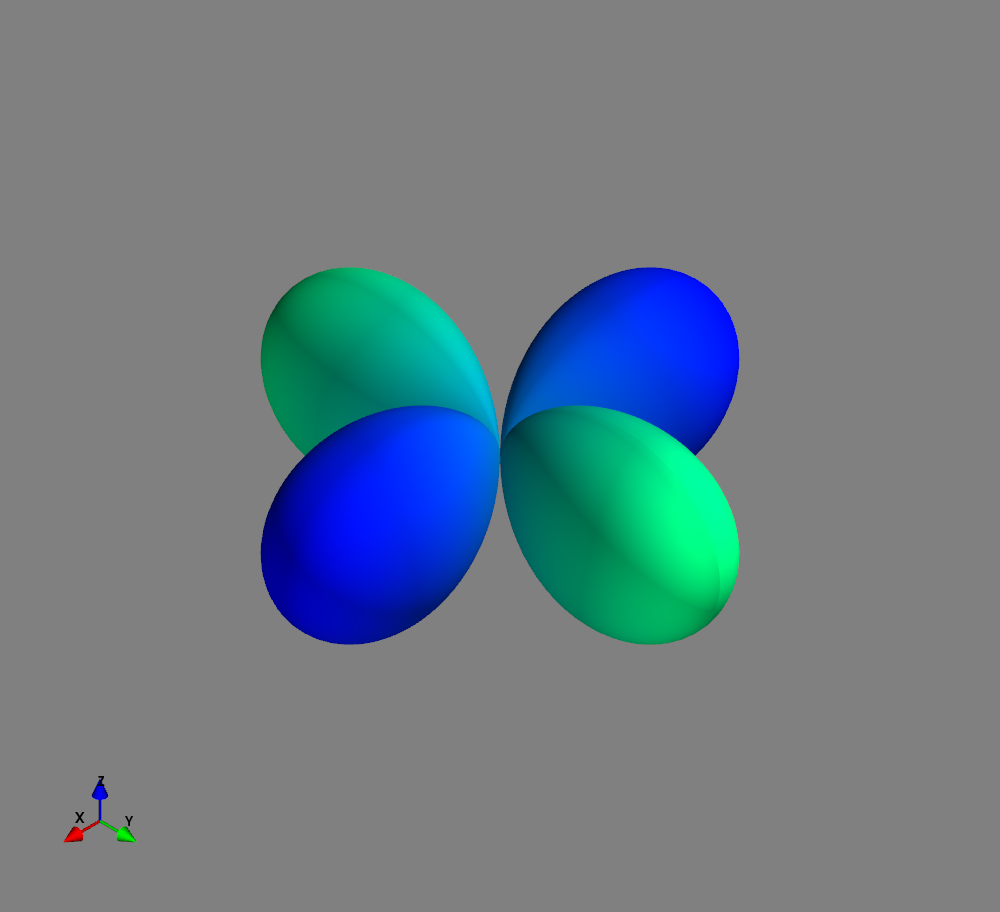
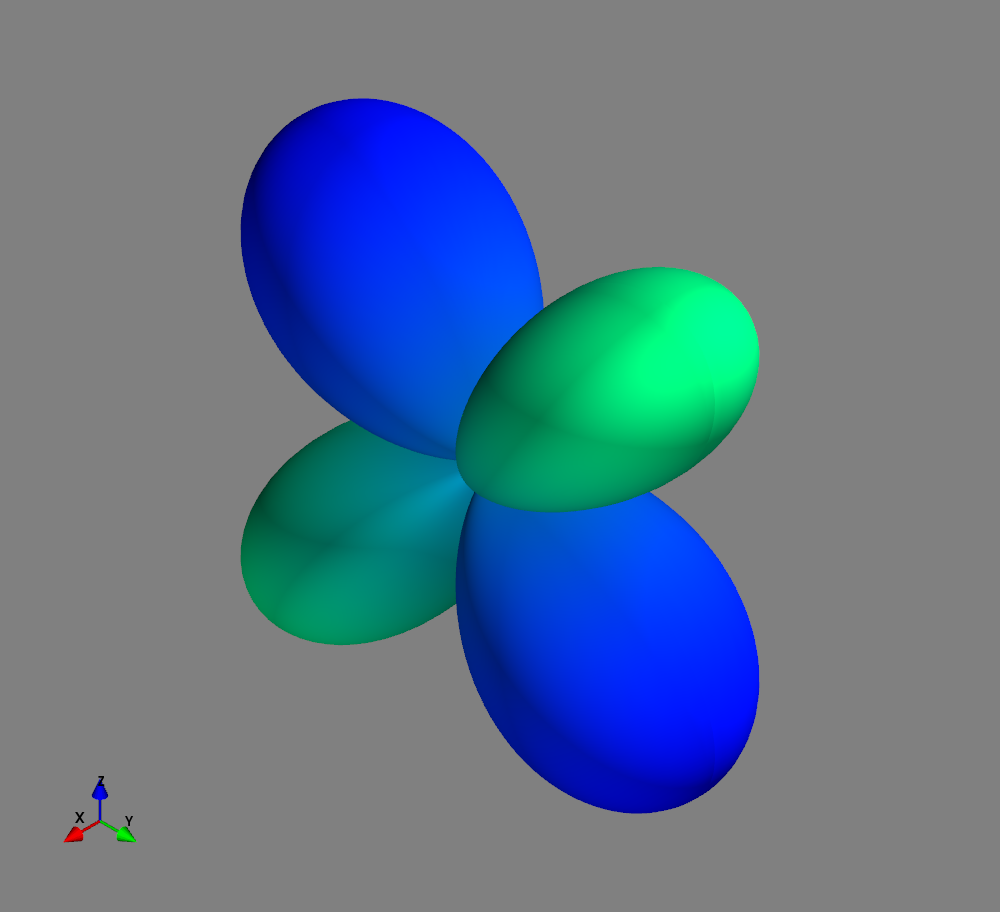
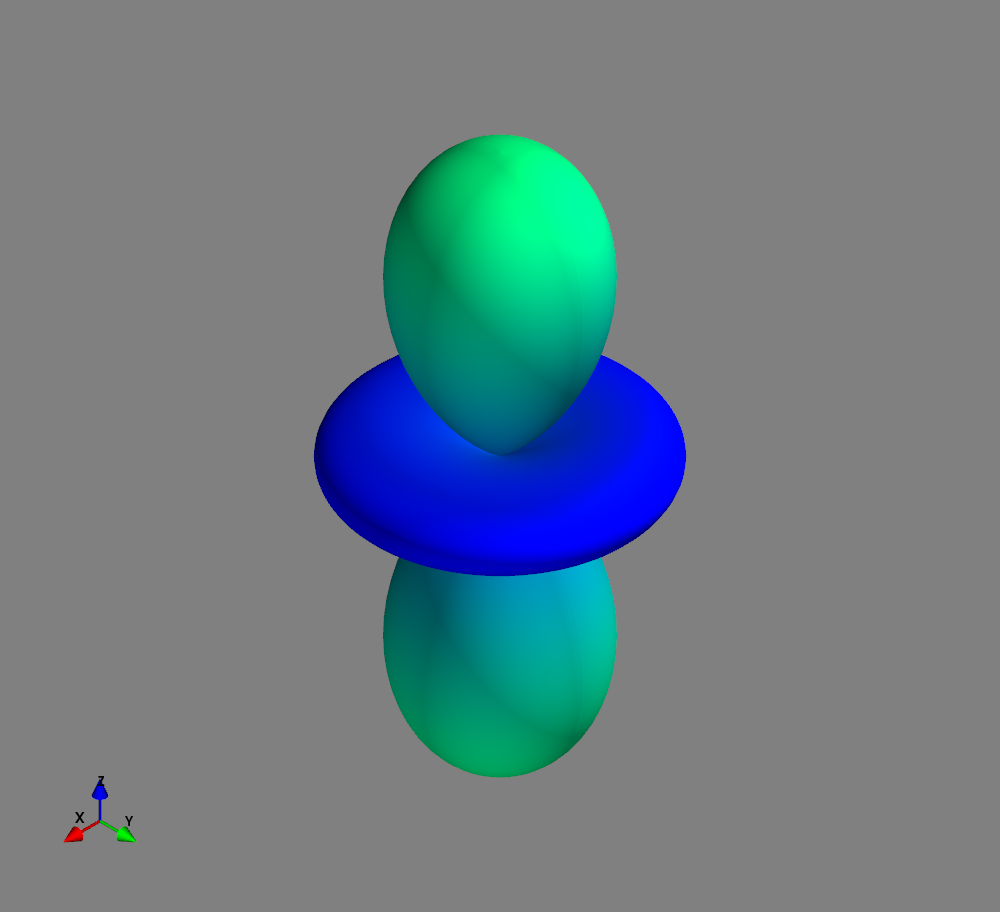
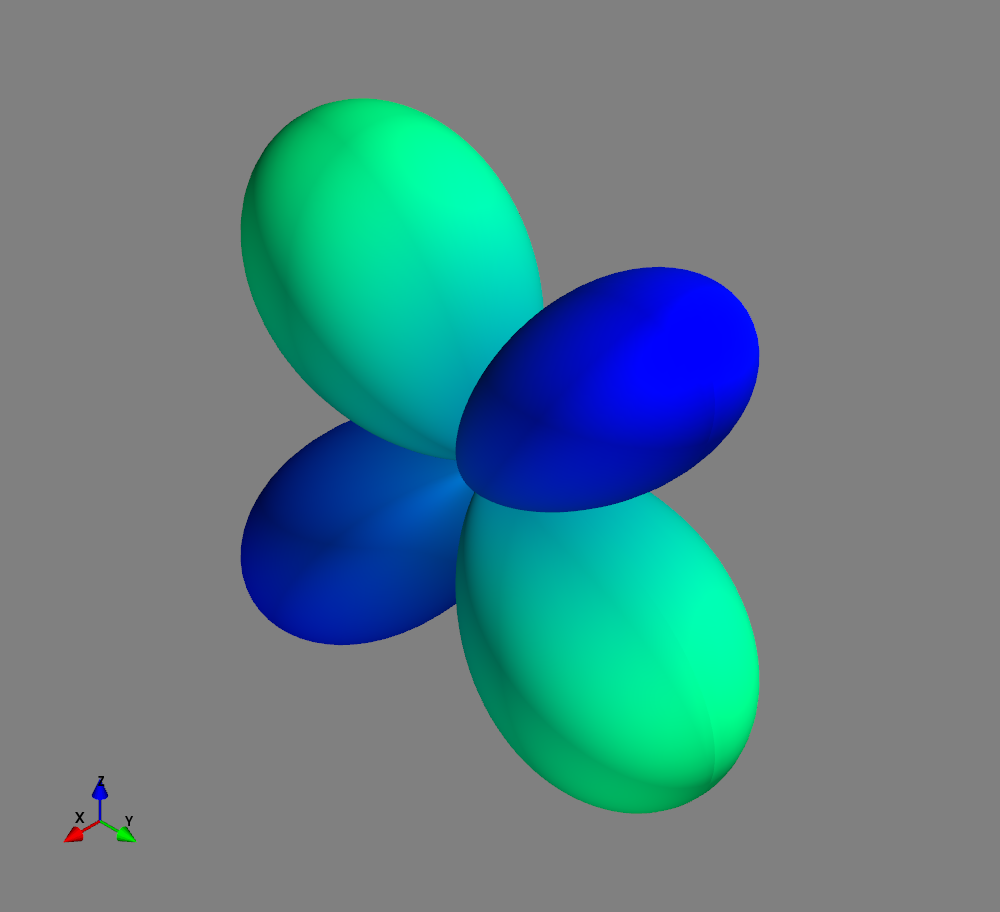
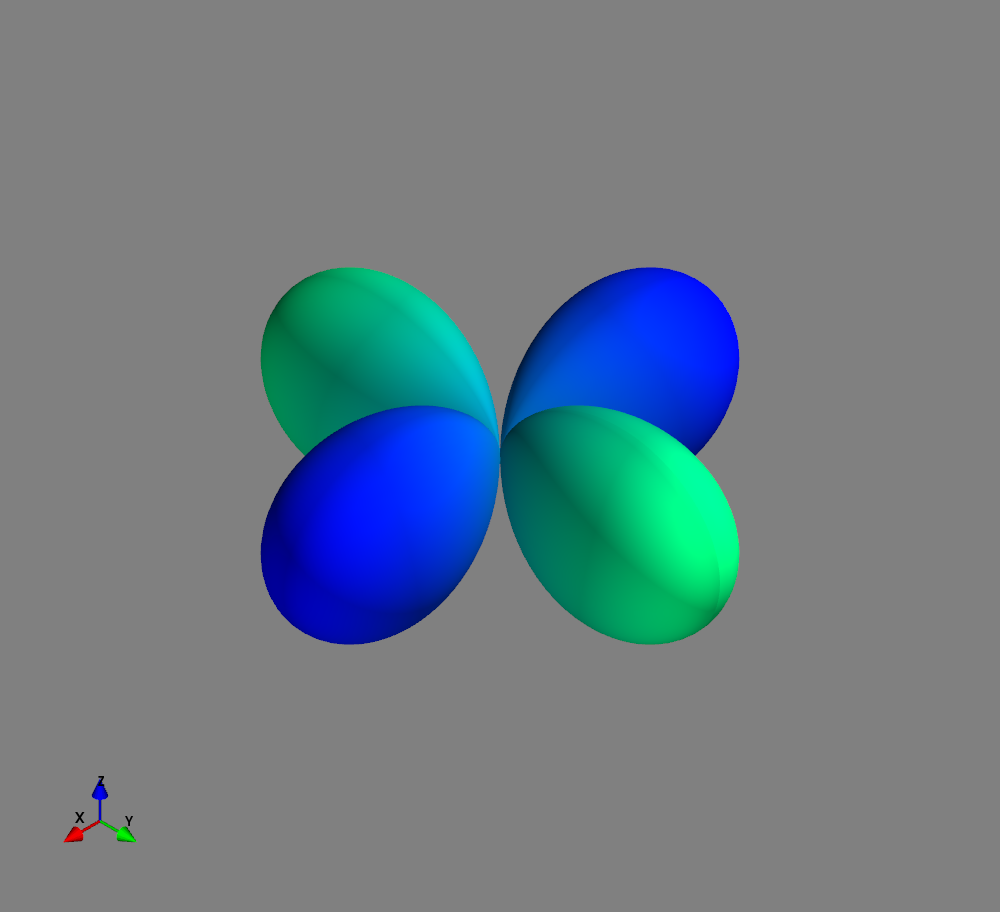
Fig.: Spherical harmonics :math:`Y_l^m left(vartheta, varphiright)` from left to the right: :math:`Y_{2}^{-2}`, :math:`Y_{2}^{-1}`, :math:`Y_{2}^{0}`, :math:`Y_{2}^{1}`, and :math:`Y_{2}^{2}`. Note the swaped colors of the dumbbells, if changing :math:`m = -1 rightarrow +1`. Changing :math:`m = -2 rightarrow +2` only affects the complex exponential function and is not visualized.
Solving the solution function
Previously we have derived the sperical harmonics and their squares as angular dependence of the probability density of a perticle within a spericaly symmetric potential. Now, we would like to solve the last dependence, namely the solution for the radial function
If we multiply this equation with
where the qunatum number
as an example for a sperically symmetric potential and replace the mass
In order to solve this equation for
were we made use of the abbreviation
With the substtution
we obtain
In order to solve this problem we use the ansatz of a power series
If we use this series in the differential equation and compare the coefficients, we obtain the recursive formula
In order to normalize
Furthermore, on the basis of the recursive formula we obtain
which leads to
The qunatization of the energy eigenstates arise from the condition
which leads to the condition for the quantum number of the angular momentum
On teh basis of teh recursicve formula w can calculate the function
The first three normalized radial functions
1 |
0 |
|
2 |
0 |
|
2 |
1 |
|
3 |
0 |
|
3 |
1 |
|
3 |
2 |
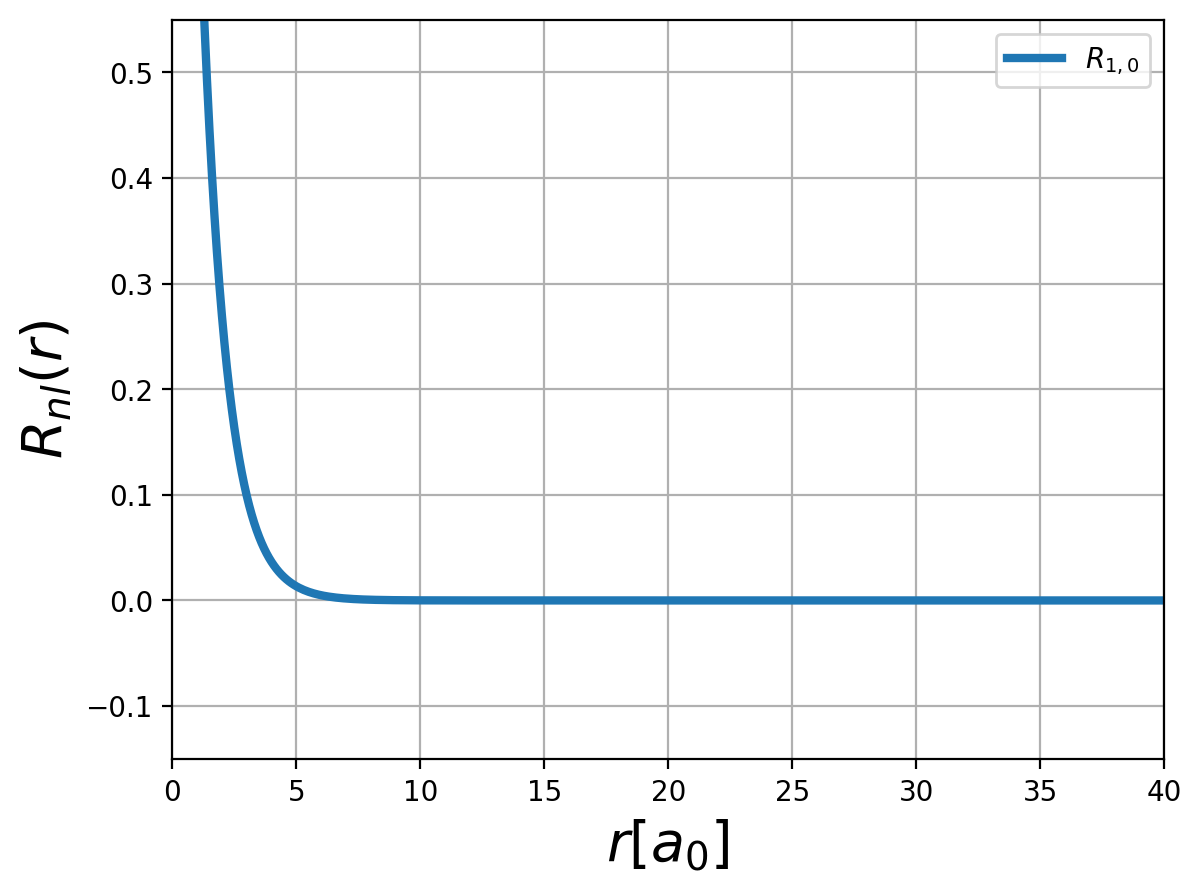
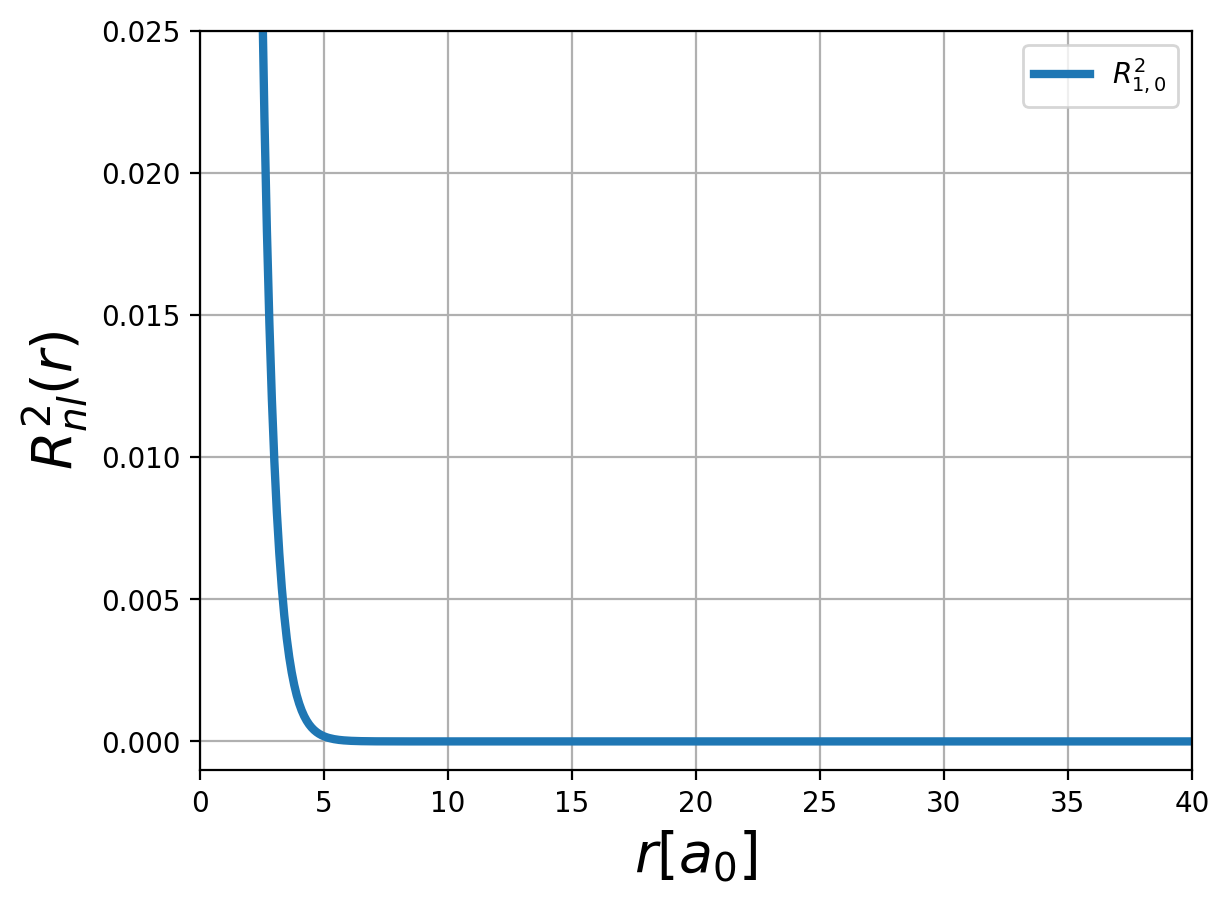
Fig.: (left) The radial function :math:`R_{n,l} left(rright)` and (right) the corresponding probability density :math:`left| R_{n,l} left(rright) right|^2` for :math:`n=1`.
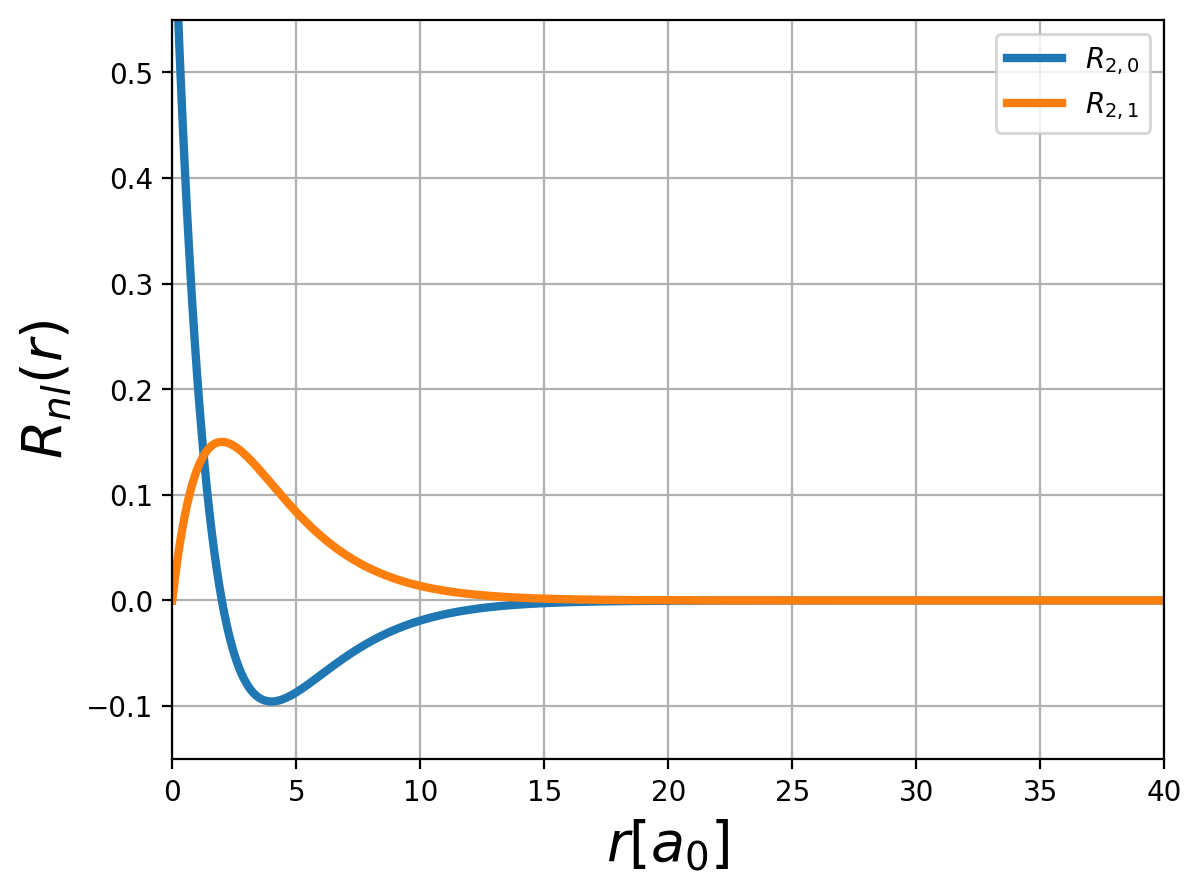
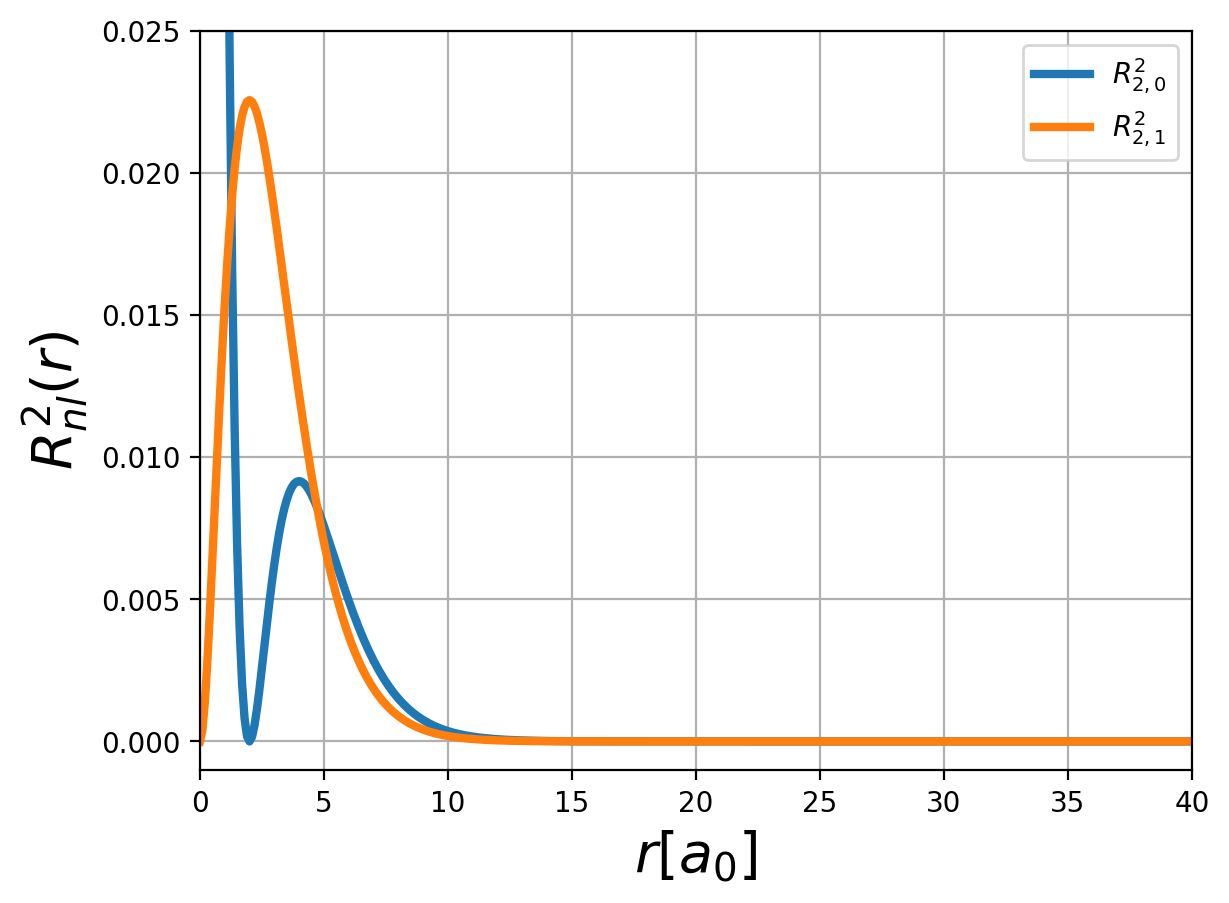
Fig.: (left) The radial function :math:`R_{n,l} left(rright)` and (right) the corresponding probability density :math:`left| R_{n,l} left(rright) right|^2` for :math:`n=2`.
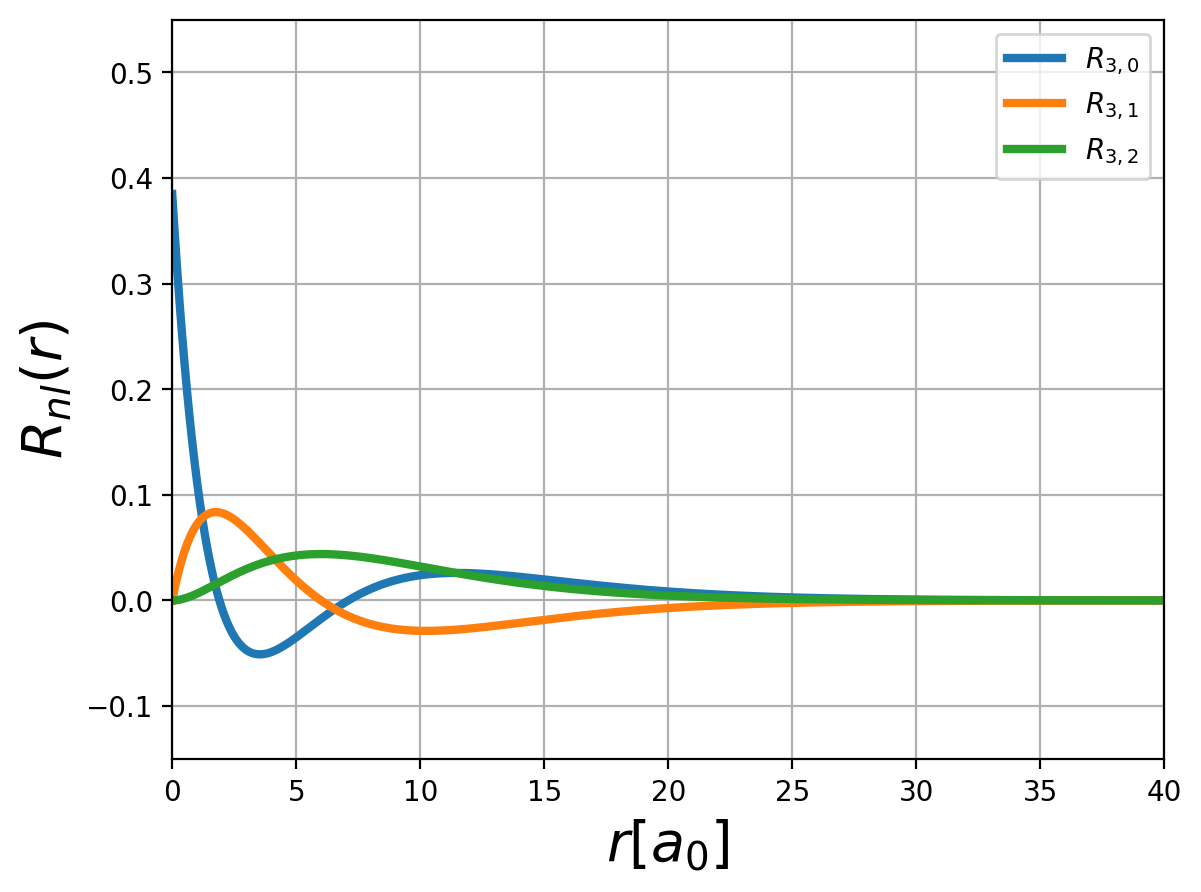
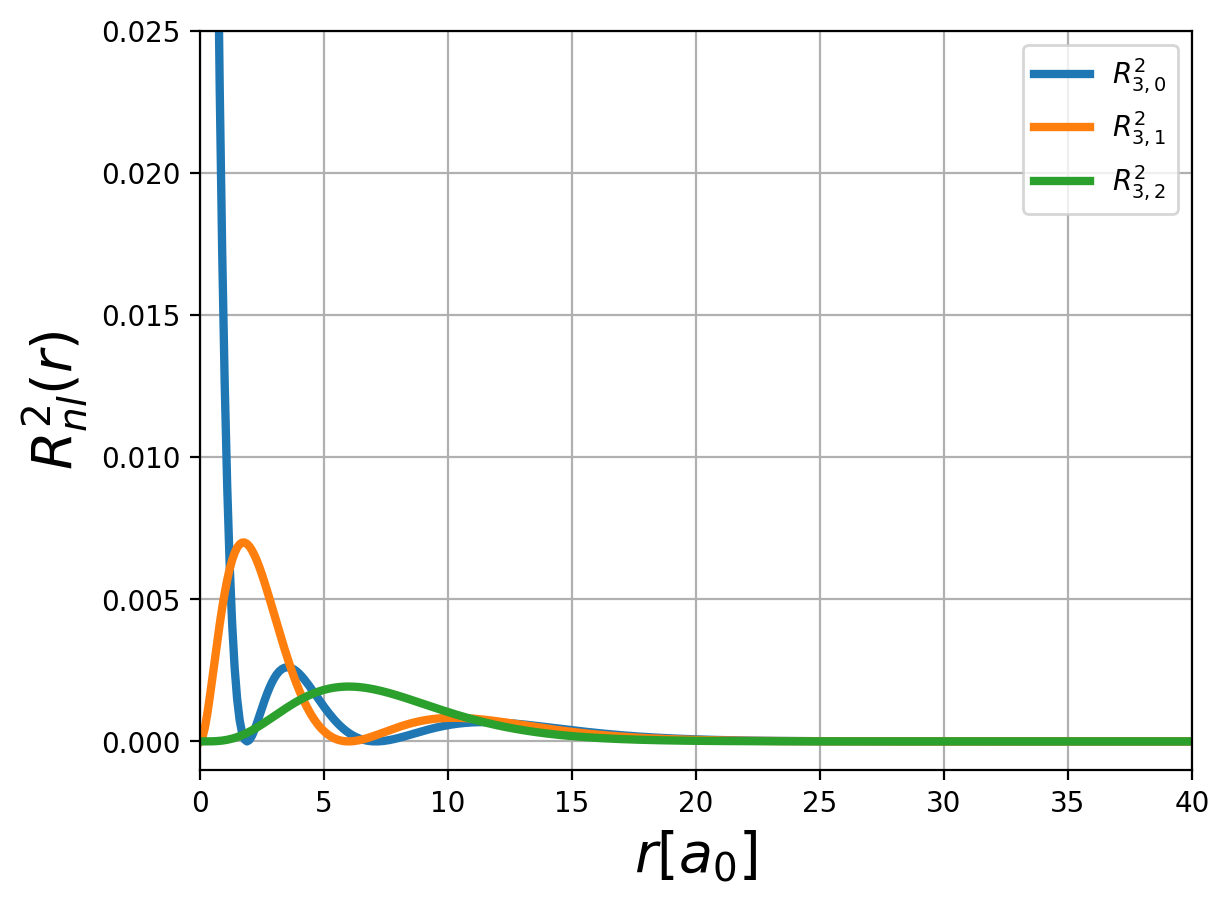
Fig.: (left) The radial function :math:`R_{n,l} left(rright)` and (right) the corresponding probability density :math:`left| R_{n,l} left(rright) right|^2` for :math:`n=3`.
We can see that the energy of a quantum state depends only on the principal quantum number
distinct states
.
