This page was generated from `/home/lectures/exp3/source/notebooks/L21_AMA/Plancks_law.ipynb`_.
The Birth of Quantum Mechanics¶
Stefan-Boltzmann Law¶
In order to derive the expression which we know today as Stefan-Boltztmann law, Stefan discovered the empirical relation in 1879 and later in 1884 Boltzmann derived the law on the basis of thermodynamics and Maxwell relations. First we want to consider the inner energy
If we now calculate the derivative of the inner energy with respect to the volume at constant temperature
and make use of one Maxwell relation, namely
we get
Previously Maxwell presented an expression for the radiation pressure being
with
being proportional to the Stefan-Boltzmann constant
Wien’s Displacement Law¶
In 1896 Wien published how the spectrum of cavity radiation changes with altered temperature. Today this law is often not refered to the overall shape of the spectrum, but rather to the maximum of the spectrum. In 1896 the Stefan-Boltztmann law was already published stating that the emitted radiance depends on the apparent temperatrure to the power of 4 (
On the basis of thermodynamic concepts and the Stefan-Boltzmann law Wien derived a relation between the wavelength
and
The last equation can be reformulated into the shape of
with
Wien further examined the integration with the result for the energy profile
Wien’s distribution law or Wien approximation¶
In his original publication in 1896 Wien employed the wavelength dependence of the blackbody radiation and the Mawell-Boltzmann distribution for the speed of molecules. On the basis thermodynamic arguments he derive a formula for the radiance
with
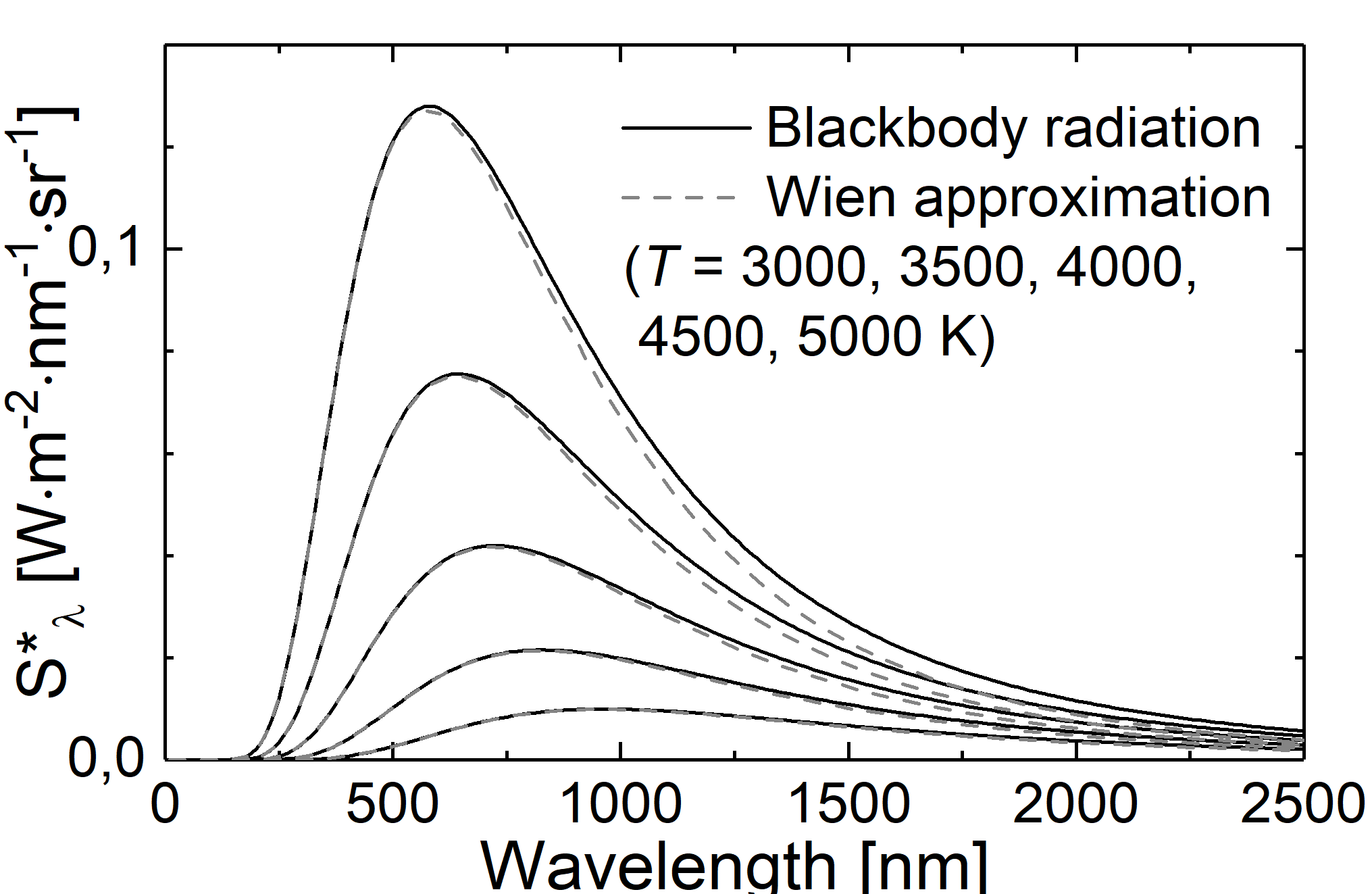
Fig.: Comparison between blackbody radiation (solid lines) and Wien approximation (dashed lines) at different temperatures.
Rayleigh–Jeans law¶
In order to calculate the average energy per eigen-oscillation Rayleigh and Jeans used the classical appraoch. Similar to the hamonic oscillators every mode bears the average energy of
with
rises quadratically with respect to the frequency
If we no consider a temperatur of about
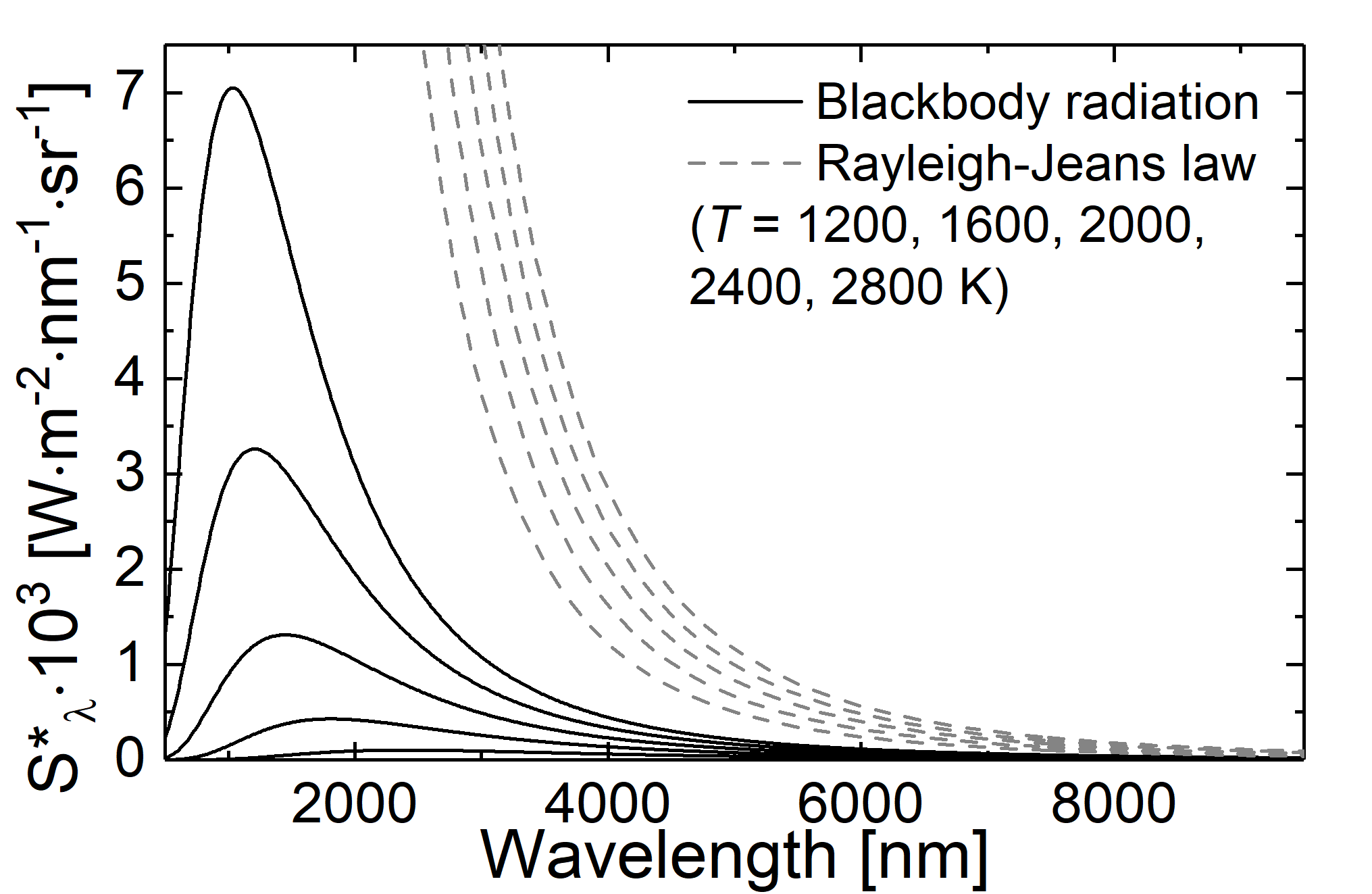
Fig.: Comparison between blackbody radiation (solid lines) and radiation as decribed through the Rayleigh-Jeans law (dashed lines) at different temperatures.
Planck’s law¶
In 1900 Max Planck faced the question how to omit the ultraviolet catastrophe and to describe the blackbody radiation as a whole.
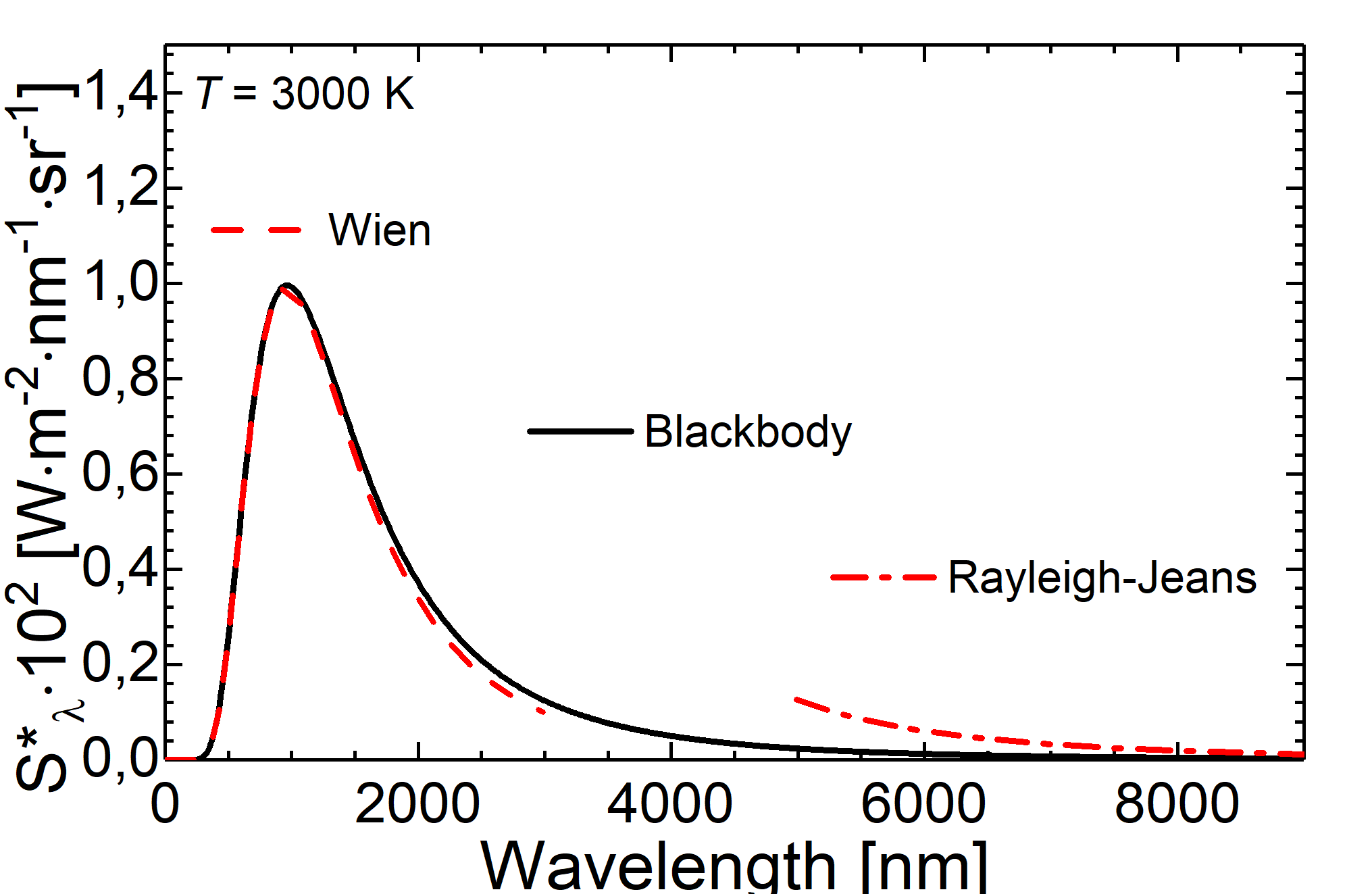
Fig.: Comparison between blackbody radiation at 3000 K (solid line) and radiation as decribed through the Wien approximation (dashed line) and the Rayleigh-Jeans law (dash-dotteded line).
He proposed a revolutionary hypothesis called Quantum Hypothesis or Planck’s Postulate. As Rayleigh and Jeans before, Planck assumed the modes within a cavity resonator as oscillations. However, in contrast to the classical approach allowing every oscillator to acquire every, arbitrary small value of energy (
The letter
This event of postulating a smallest quantum of energy is often referred to as the birth of quantum mechanics. Nowadays we can define the smallest quantum of the electromagnetic field bearing the energy
If we now consider thermal equilibrium, the likelyhood
Since we did calculate a likelyhood, the relation
The spectral energy density of a cavity radiator then is given through
which leads us to the famous Planck’s formula
Here
So far we have expressed Planck’s law in dependece of the frequency
and the radiance
as functions of
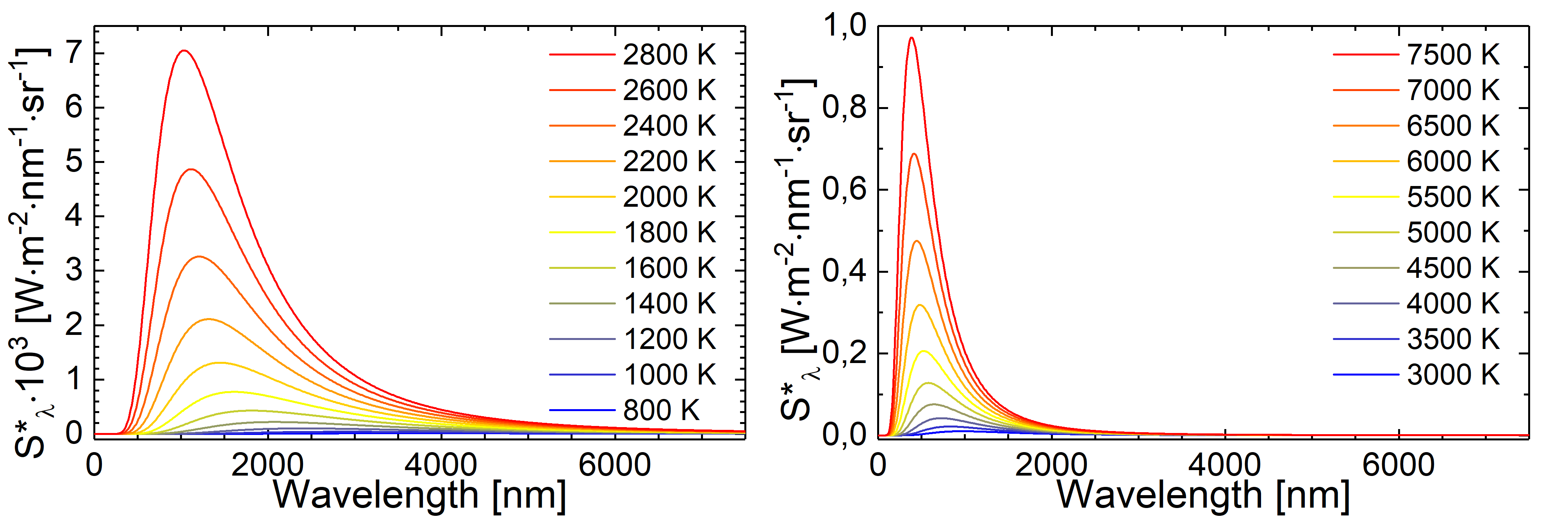
Fig.: Blackbody radiation as described through Planck’s law of radiation at different tempertures.
Rayleight-Jeans law as special case of Planck’s law¶
Now we consider Plank’s law in dependence of the frequency and invstigate this formula in the case of small energies with
As a consequence we get
and
Here we demonstrated that the Rayleigh-Jeans law is a special case of the general Planck’s law for the case of small energies (
Wien approximation as special case of Planck’s law¶
Now we consider Plank’s law depending on the wavelength and study this formula in the case of high energies with
As a consequence we get
and
If we know compare these equations with Wien’s distribution law
we are able to identify
Here we demonstrated that Wien’s distribution law is a special case of the general Planck’s law for the case of high energies (
Wiens displacement law as derived from Planck’s law¶
In order to determine the spectral position (
then leads to the result
Because the ratio
Here the product
where we can identify the parameter
It is not supprising that
into account during switching the domains (
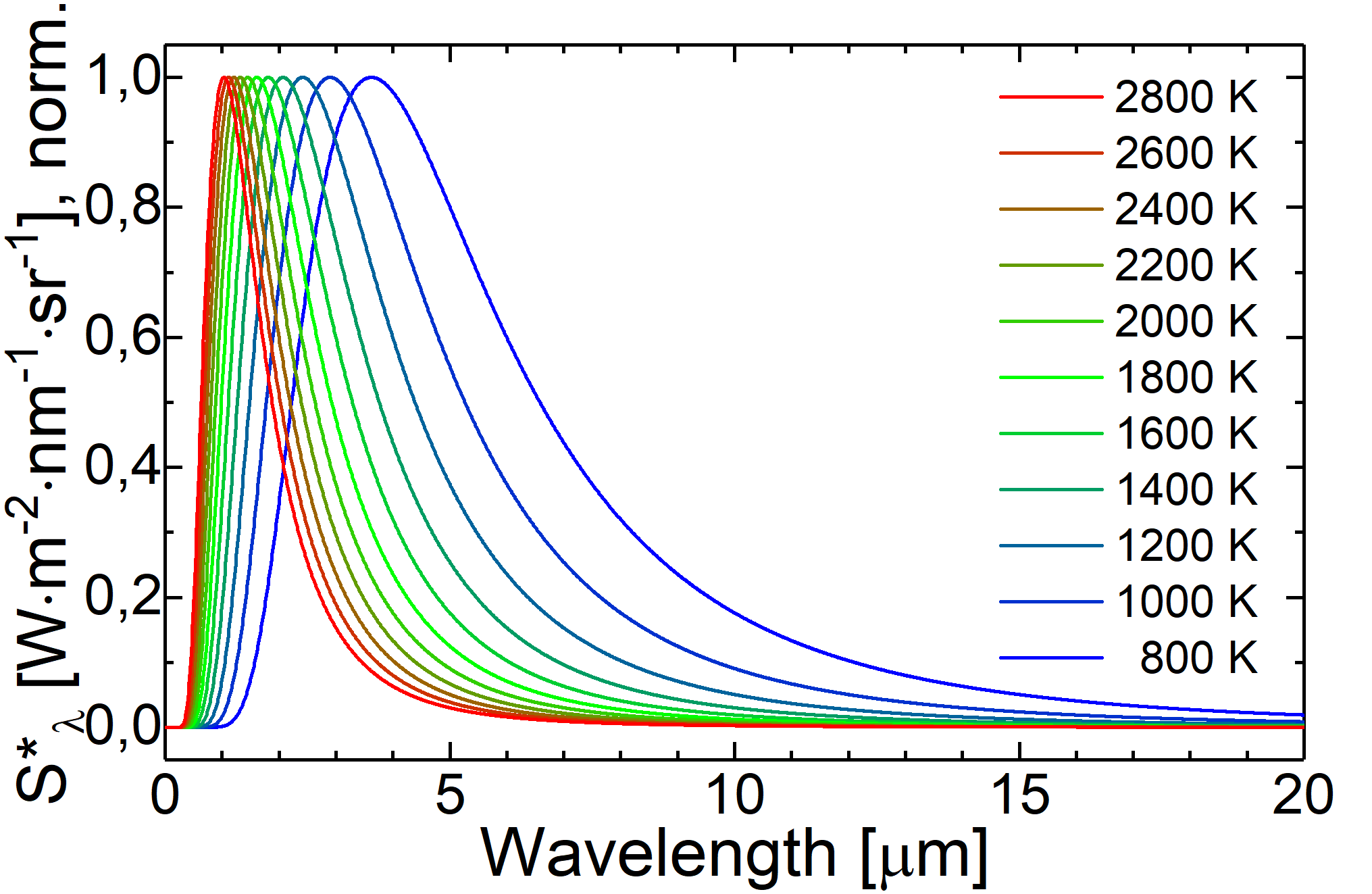
Fig.: If we normalize the spectral radiancy from Planck’s law, the shift of the maximum position becomes eveident.
Stefan-Boltzmann law as derived from Planck’s law¶
As initially proposed by Stefan and Boltzmann on the basis of thermodynamic considerations, the Stefan-Boltztmann law can be derived as well on the basis of Planck’s law. Therfor, we integrate the energy density of the cavity radiation over all frequencies
If we apply the substitution
we derive
with
which is the Stefan-Botzmann law.
If we now concentrate on the radiance which is emitted from an area element
we can calculate the radiant power which is emitted from one hemisphere as
Thus, we were able to relate the Stafen-Boltzmann constant
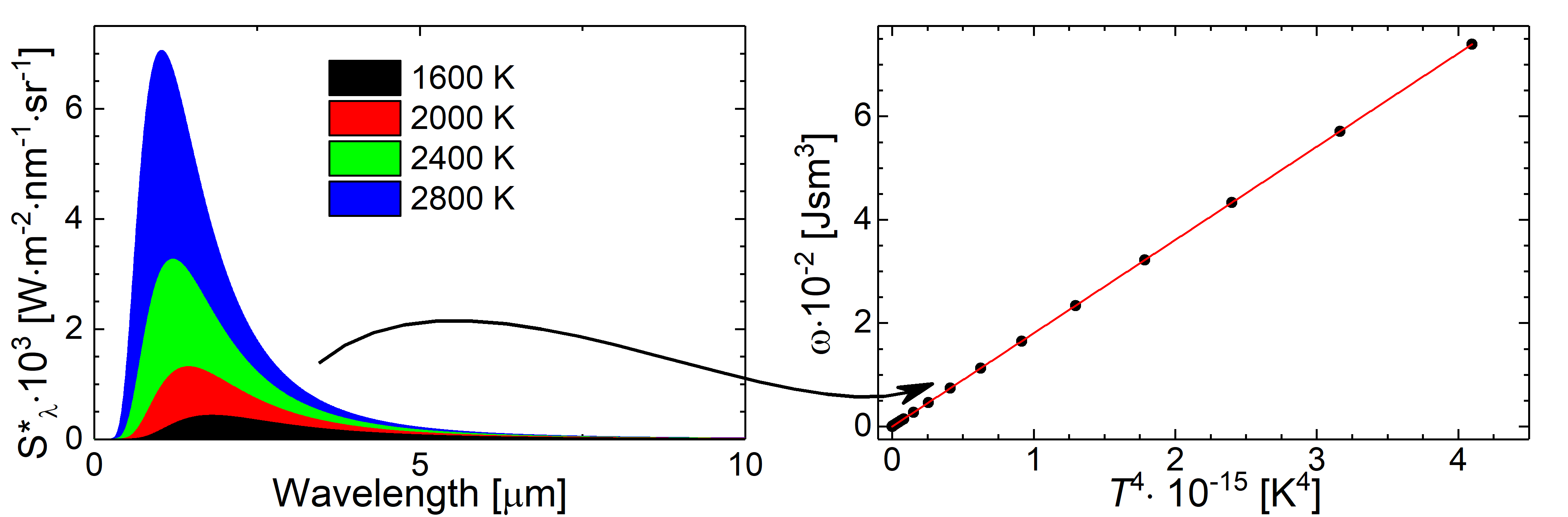
Fig.: If one calculates the energy density as integration over the spectral energy density :math:`int_{nu / labmda =0}^{infty} mathrm{d} nu / lambda , omega left( nu / lambda , Tright)` or the power density as integration over the spectral radiancy :math:`int_{nu / labmda =0}^{infty} mathrm{d} nu / lambda , S^{ast}_{nu / lambda} left( nu / lambda , Tright)` (both differ only by a constant factor), the Stefan-Boltzmann law can be demonstrated.
On the basis of these exmaples we could successfully correlate empirical findings to Planck’s law and, moreover, relate empirical constants like
