This page was generated from `/home/lectures/exp3/source/notebooks/L19_AMA/Structure_of_matter.ipynb`_.
The Structure of Matter - a Historical Approach¶
Do atoms exist?¶
We would like to briefly introduce historic experiments facing the question, whether atoms really exists. Therefore, we will discuss Dolaton’s law and the law of Gay-Lussac before we resolve the structure of atoms an the basis of Thomson’s and Rutherford’s experiments.
The oldest documents about the structure of matter being composed of infinite small, but space-filling particles originate from the Greek philosophers Leukip and Demokrit. Those particles differ in size and shape, and properties of objects on the macroscopic scale arise from the arrangement of these particles. Since they assumed these particles are indivisible, they named them “:math:`alpha tau o mu o zeta`” (atoms). One century later,Epikur* refined the picture of atoms through ascribing the characteristic of mass to these particles, in addition to space demand.
Atoms and molecules¶
Dalton’s law¶
On the basis of precise quantitative analyses of chemical reaction and the ratios of educts and products, John Dalton developed the idea that every chemical compound is composed of substances, whereas the ratio of these substance is constant for a particular compound and the ratio unambiguously identifies a compound. In 1803 Dalton developed his idea of a chemical reaction being the separation and association of atoms, and published this idea in 1808 “A New System of Chemical Philosophy”. This publication stated three postulates: - All chemical elements are composed of small particles which cannot be dismantled any further. - All atoms of the same chemical element are identical in quality, size, and mass. However, these properties differ from the according properties of other elements. Consequently, the characteristics of an element are governed through the properties of its atoms. - If chemical elements form a chemical compounds, their atoms associate in a ratio of integers.
The law of Gay-Lussac¶
In 1805 Joseph Louis Gay-Lussac and Alexander von Humboldt discovered that hydrogen and oxygen at the same (partial-) pressure associate to water if they use volume ratios of 2:1, respectively. Later Gay-Lussac published: “If two or more gaseous substances associate at equal pressure and equal temperature, their volumes have an integer ratio”.
The Avogadro number¶
In 1811 Amadeo Avogadro introduced the term “molecule” as smallest particle of a gas which has the same characteristics as the gas itself. As a consequence Avogadro stated that at identical pressure and temperature equally big volumes of different gasses contain the same number of molecules. On the basis of his experimental findings, the term “molar volume” was introduced. The first definition was molar volume is the volume of 1 mol of a gas at standard conditions
(
These experimental findings and concepts pathed the way for the modern understanding that mater is generally composed of atoms.
The Structure of atoms¶
Indication of charged particles within atoms¶
At the end of the 19th century experimental findings accumulated indicating matter bears charged particles. The main results were: - Experiments on electrolytic current demonstrated that molecules can dissociate, whereas the resulting ions migrate in opposite directions and transport charges and mass. - Gas dicharging phenomena are influenced through electric and magnetic fields. Thus, discharging is correlated to motion of charged particles. - Magnetic phenomena arise from electric conduction
in metals and semiconductors. -
Johann Wilhelm Hittorf observed in a gas discharge tubes that particles emitted from a cathode propagate at straight lines. Moreover, these particles can be deflected with the aid of a magnet. As a consequence of the emission from the cathode and the direction of the magnetic poles, these particles had to be negatively charged. Later in 1897, Joseph John Thomson determined the charge-to-mass ratio
In 1899 Thomson and Charles Wilson studied sinking droplets of condensed water vapor. The speed of falling was depending on the size of the droplets and the viscosity of the retarding gas. While measuring the amount of water and charges Thomson and Wilson were able to estimate the elementary charge of about
Concerning the mass of an electron, its value is still only accessible via the charge-to-mass ratio. A precise measurement of the
The Thomson model¶
At the beginning of the 20th century only negatively charged electrons as cathode rays and positively charged
As a first attempt, in 1904 Thompson proposed the “Plump Pudding Model”. According to this model every atom consists of a number of
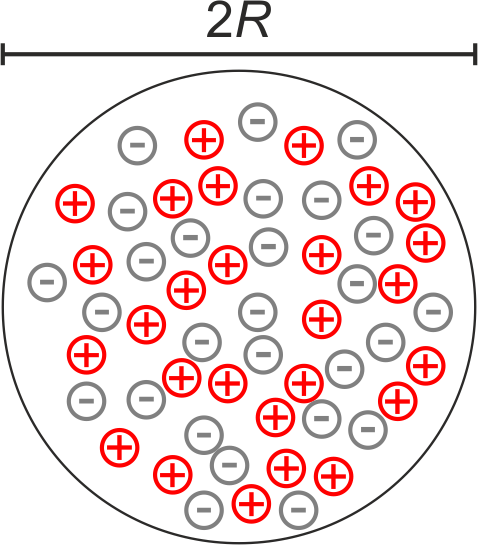
Fig.: Model of an atom as proposed by Tomson. The equal amount of positive and negative charges are distributed across the atom volume.
Indication of a not-sufficient description is provided on the basis of simple calculations. If the
Then, the plasma frequency would result in
However, the estimated absorption and emission frequencies did not coincide with experimental results.
The Rutherford model¶
In 1909 Rutherford, Geiger and Marsden conducted scattering experiments of
In order to calculate how the
which is determined through the density of scattering centers
Concerning scattering on a Coulomb potential with the point-like charge
with
we make use of the definition of the differential scattering cross section
and calculate the derivative of
When making again use of the relation between the scattering angle
If we use
which is the Rutherford formula. This formula reproduced the measured data except for big angles, and thus small impact parameters. Rutherford concluded that deviations at big angles arise from the nuclei which are in fact small compared to an atom but not point-like.
