This page was generated from `/home/lectures/exp3/source/notebooks/L25_AMA/L25_structure_of_atoms.ipynb`_.
The Structure of Atoms and the Bohr Model¶
So far we have discussed the models of the structural organization of atoms as proposed by Thomson also called Plum Pudding Model and proposed by Rutherford. We know that charges are not equally distributed across the volume of the atom (as assumed in the plum pudding model). Positive charges are rather comprised within a tiny volume, whereas negative charges form a shell around teh positive core. In detail, positive charges form the core with a diameter of
Now, we will discuss how the atomic shell might be structured. One question is, whether the distribution of electrons is a static distribution of charges or whether the electrons move. The first case is not stable, because of the electrostatic attraction between the positive nucleus and the negative electrons. The second case, however, is puzzling as well, because accelerated and decelerated charges are supposed to emit electromagnetic waves which is not observed.
Atomic sprectra¶
In 1859 Kirchhoff and Bunsen observed that atoms absorb light only for particular values of the wavelength. Moreover these wavelength values are specific for the particular kind of atoms and form the absorption and emission spectra of these atoms. Early instruments for recording spectra made use of a prism as diffracting element and a photo plate for recording. The prism gave rise to a rainbow-colored stripe at the plate and narrow lines of reduced intensity appeard exactly at those positions that correspond to the particular wavelengths of atom absorption. Thus, the negative of the photo plates showed bright lines at these positions and those compositions of lines were called line spectra. On the basis of further experiments one was able to state
For every wavelength at which an atom absorbs emission of light is possible, if the atom was previously supplied with enough energy
The absorption and emission spectra are specific for every kind of atoms. Thus, one is able to determine the chemical element absorbing or emitting the radiation on the basis of the line spectrum.
The values of wavelength (spectral lines) are not arbitrarily narrow. They rather exhibit a distribution of intensity. This indicates that atoms do not emit monochromatic radiation.
In 1885 Balmer discovered that the emission spectrum of hydrogen is compossed of a series of lines that obey a specific law, namely
In this equation
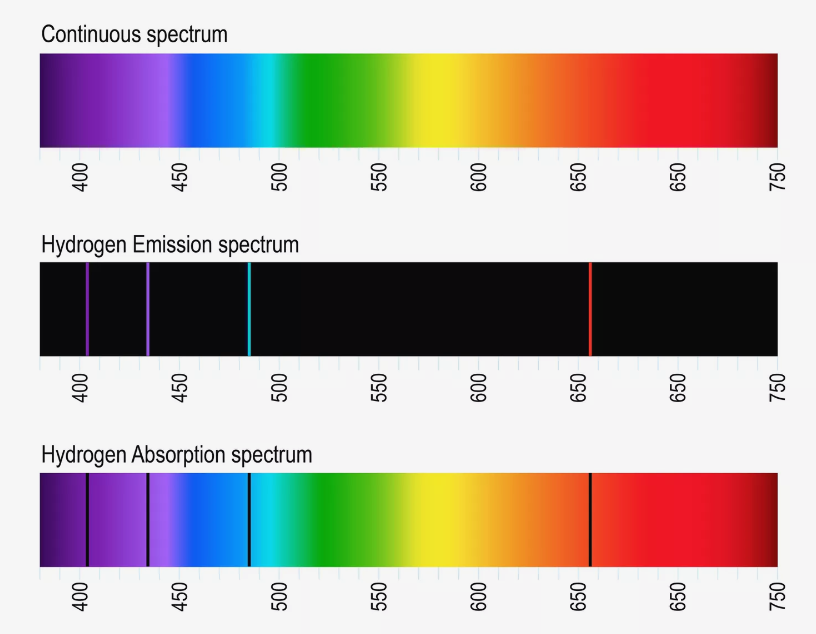
Fig.: (top) A continuous spectra in the visible range of light. (center) A hydrogen emission line spectrum. Only where hydrogen emitts light narrow lines of light appear. (bottom) A hydrogen absorption spectra. At wavelengths where hydrogen absorbs light the remaining intensity drops and black lines appear. Source: https://www.thoughtco.com/definition-of-balmer-series-604381
The Bohr model¶
On the basis of the spectral lines, a number of models for the architecture of atoms were porposed but they were not able to explaine all experimental results at a whole. Among them were Bohr’s famous model from 1913. In the framework of this model electrons with mass
we can determinethe radius of the orbital
As long as there are no constraints for the energy of the electron
with
Now, we can use the expression for the velocity
The constant
represents the Bohr radius, which is the smalles radius of the electron orbit (
Concerning the kinetic energy of an electron
We can see that the kinetic energy of an electron is equal to
We can further use the expression for the discrete radius
with the Rydberg energy (Rydberg constant with respect to energy)
It is evident that the electron can adopt only particular values of energy
Moreover, it is evident that the energy is negative and approaches
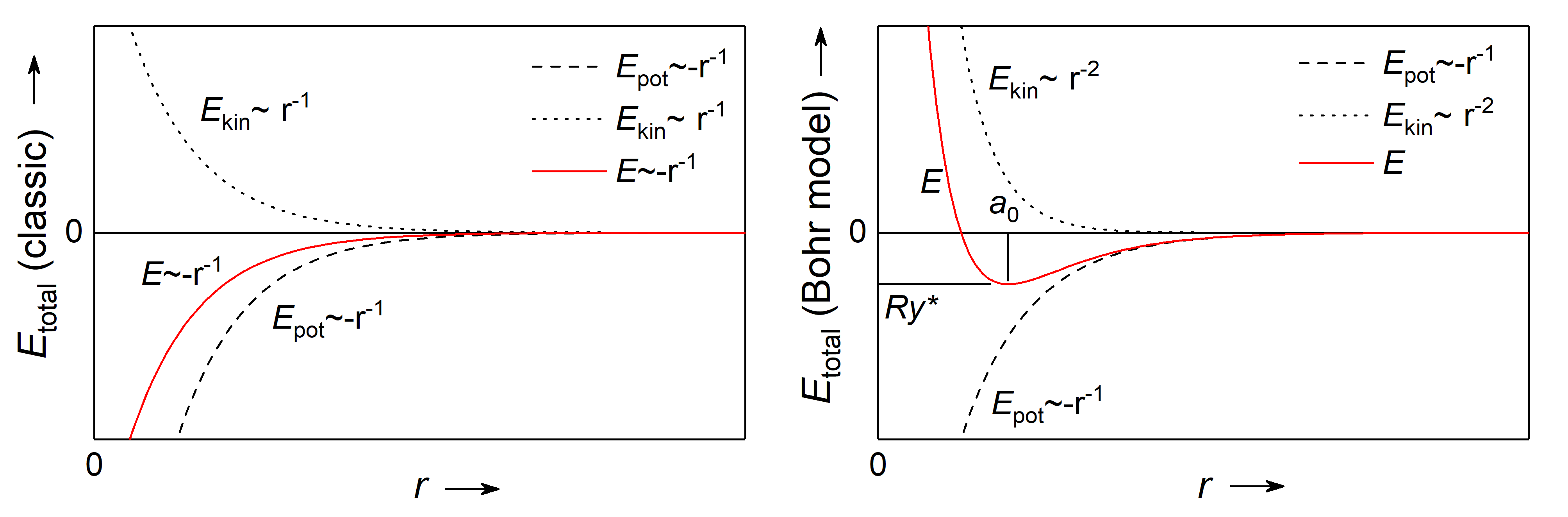
Fig.: (left) Kinetic energy, potentual energy, and the resulting total energy as sum of the fomer of an electron within the Coulomb potential in the framework of the classical mechanics and electrodynamics in dependence of the distance of the lectron from the nucleus. (right) The same is left, but the quantum mechnical correction resulting in a minimum of the total energy.
Please note:
The particular value of the Ryberg constant depends on the reduced mass of the system electron plus nucleus
The Bohr model is a semi-classical model. The motion of the electron is treated in the framework of classical mechanics as the motion of a point-like particle within a Coulomb potential plus the additional constraint from the matter wave leading to quantized quantum states.
From the quantized velocity of the elctron
In order to explain the shape of the line spectra we have to state a hypothesis. If a photon is absorbed by an atom, then the atom might go from a lower energtic state
The energy of the photon is used to compensate the energy difference between state
With the aid of
which is the general equation for the lines of a line spectrum and comprises the equation stated by Balmer for special the case of hydorgen (
In summary we can state:
Electrons propagate in circular orbitals around the nucleus. The radii of the orbital are quantized and the particular radius increase quadratically with the principal quantum number
The radii are inverse propertinal to the atomic number.
Every quantum state characterized through the quantum number
The energy for
Through absorbing a photon of energy
So far the Bohr model can successfully describe the line spectra. However, since electrons are assumed to propagate at circular orbits, they are supposed to permanently irradiate electromagnetic waves. This gives rise to the questions why does the electron not emit radiation and therefore lose energy and collapse into the nucleus? Or why are atoms stable?
About the stability of atoms¶
The stability of atoms in its lowest energetic state can be explained on the basis of the uncertainty principle. If we assume
since we know the electron has to be somewhere within the atom. As a consequence the radial component
Because of that, the overall electron momentum has to bigger than the uncertainty
otherwise we would know the momentum with a smaller uncertainty than
and
respectively. The total energy
The propability to find an electron at the radius
As a consequence, there is a state of minimum energy with the lower limit
This result is in accord with observation from the Bohr model stating the energy of the lowest state in a hydrogen atom is
